Abstract
Multiple Myeloma (MM) progression and drug resistance depend on bidirectional interactions with the local microenvironment that results both in a destruction of the marrow and survival of the tumor. Similarly to how MM cells both depend on and destroy (or render dysfunctional) BM mesenchymal stromal cells (MSCs), we hypothesized that MM cells also affect and are affected by BM adipocytes (BMAs), contributing to disease progression. Obesity, which is a risk factor for MM development with overweight individuals being 1.5-2 times more likely to develop the disease, also correlates to increased bone marrow adipose tissue (BMAT). Similarly, correlations between aging and BMAT, as well as aging and MM exist. These correlations suggest a role for BMAs in the development and progression of MM and make elucidating the signals and cross-talk between MM and BMAT extremely relevant in the understanding of the disease. BMAT-derived signaling molecules may promote myelomagenesis and enhance tumor growth, but this has been largely unexplored. In addition, while the effect of myeloma on bone cells has been widely characterized within the past few years, the direct effect of MM cells on BMAs is unknown and potentially revolutionary for our understanding and treatment of MM.
In the present study, we explored the direct effects of MM cells on adipocytes and BMAs in 2D and novel 3D tissue engineered cultures. 3T3-L1 pre-adipocytes or primary mouse or human BM-MSCs were differentiated into lipid-laden adipocytes using previously published methods, and co-cultured with MM cell lines (MM.1S, MM.1R, 5TGM1, and OPM2). For mature adipocytes, cells were differentiated for approximately 9 days (mouse) or 21 days (human) prior to myeloma co-culture. To investigate the effect of myeloma cells on MSC adipogenic differentiation capacity, pre-adipocytes were grown to confluency and then myeloma cells were added for a 3-day exposure period prior to adipogenic differentiation. Direct and indirect (MM conditioned media (CM) and transwell cultures) effects of myeloma cells on MSC adipogenesis were assessed with oil red-o functional assays for lipid accumulation and/or qRT-PCR of total adipocyte RNA. Statistical significance was determined via Student's T-test or ANOVA. We also developed a novel, tissue-engineered (TE) 3-D in vitro MAT model using silk scaffolds seeded with MSCs grown in adipogenic media. TE-MAT was validated to represent normal, healthy MAT using confocal imaging and microarray. MM cells (MM.1S, 5TGM1 and OPM2) were cultured on TE-MAT for up to 2 weeks and examined with confocal microscopy; lipid accumulation was detected via Oil Red-O staining in these cultures.
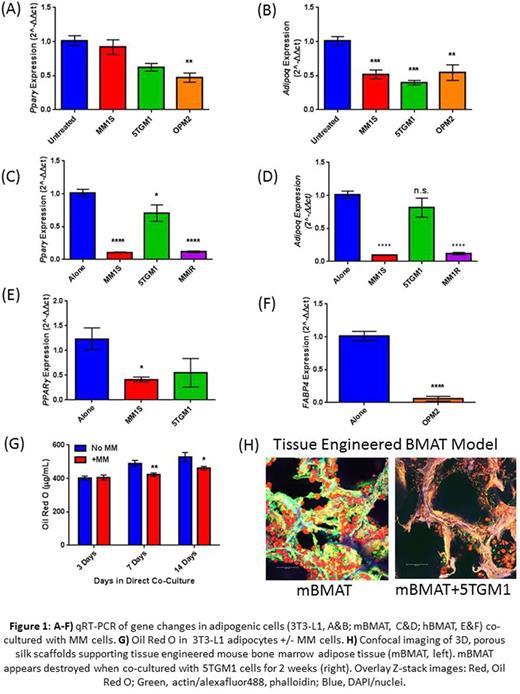
Indirect co-culture of 3T3-L1 adipocytes with myeloma cells severely and significantly reduced both adipogenic transcription factors (Fig. 1A- Ppar γ, OPM2 p<0.01; Cebp α MM.1S p<0.01, OPM2 p<0.0001, 5TGM1 p<0.001) as well as mature adipocyte markers (Fig. 1B- Adipoq, MM.1S p<0.001, 5TGM1 p<0.001, and OPM2 p<0.01). This effect was also observed in BM-MSCs differentiated into adipocytes from mouse (Fig. 1C- Ppar γ, MM.1S p<0.0001, 5TGM1 p<0.05, MM.1R p<0.0001; Fig. 1D- Adipoq, MM.1S p<0.0001, MM.1R p<0.0001), and human donors (Fig. 1E- PPAR γ, MM.1S p<0.05; Fig. 1F- FABP4, OPM2 p<0.0001), suggesting a common mechanism of MM-induced inhibition between peripheral adipocytes and BMAs. Lipid accumulation was also reduced in 3T3-L1 adipocytes in direct co-culture with MM.1S cells for 7-14 days. The trend for this effect was apparent beginning at 5 days and significant at later time points (Fig. G- 1 week p<0.01; 2 weeks p<0.05). Similarly, preliminary results showed decreased adipogenic potential from MM patient-derived MSCs vs normal controls in oil red-o staining. Co-culture with myeloma cells reduced the expression of genes involved in lipogenesis and fatty acid oxidation in both adipocyte cell types including: Srebf1 (mMAT, MM.1S p<0.0001, 5TGM1 p<0.001), Cpt1 (3T3-L1, MM.1S p<0.001), Fasn (3T3-L1, MM.1S p<0.0001) suggesting a suppression of adipogenic function as well as phenotype. Importantly, similar delipidation was also observed in TE-MAT co-cultures with myeloma cells (Fig. 1H). These combined results indicate an adverse effect of myeloma cells on adipocytes, and illuminate the BMA as a new member of the complex microenvironmental milieu in which the myeloma cell thrives.
Falank: BioPact Ventures, LLC: Research Funding. Reagan: BioPact Ventures, LLC: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal